Abstract
Introduction: Hemophilia B is a bleeding disorder characterized by coagulation factor IX (FIX) deficiency and is associated with symptoms of prolonged bleeding upon trauma and spontaneous bleed events. The current standard of care for people with hemophilia B is prophylactic FIX replacement therapy for the prevention of bleed events (Goodeve et al. J Thromb Haemost. 2015; Saini et al. Haemophilia. 2015; Castaman. Expert Rev Hematol. 2018). The aim of this study is to present a descriptive post hoc analysis of spontaneous bleed events in the context of treatment regimen (eg, prophylaxis [PPX] versus on-demand [OD]) in previously untreated participants (PUPs) with hemophilia B treated with extended half-life recombinant factor IX Fc fusion protein (rFIXFc, Alprolix) from the PUPs B-LONG study.
Methods: The PUPs B-LONG study (NCT02234310) enrolled 33 previously untreated male participants <18 years of age with hemophilia B (≤2 IU/dL [≤2%] endogenous FIX activity), with a median (range) age of 7.2 (0.96-24.0) months; most subjects (n=26 [78.8%]) were <1 year of age (Nolan et al. Blood Adv. 2021). The primary objective of PUPs B-LONG was to evaluate the safety of rFIXFc in PUPs with hemophilia B. Secondary objectives included assessment of efficacy of rFIXFc for the prevention and treatment of bleed events in PUPs (Nolan et al. Blood Adv. 2021). The primary endpoint was occurrence of inhibitor development. During the study, investigators could start a participant OD prior to PPX in accordance with local standards of care; the recommended starting PPX regimen was 50 IU/kg rFIXFc once weekly (Nolan et al. Blood Adv. 2021). Post hoc analyses included describing individual participant treatment regimen patterns on study and timing of first spontaneous bleed event by age during the study, median time to first spontaneous bleed event, and Kaplan-Meier (KM) survival analyses of time to first spontaneous bleed event by treatment regimen on study and by type of PPX treatment (ie, primary PPX versus switch to PPX). Time to first spontaneous bleed event was calculated by subtracting the start date of the bleed event and the start date of regimen, plus 1. Participants were considered to have received primary PPX treatment if they began PPX at the start of the study; the switch PPX regimen included participants who switched from OD while on study.
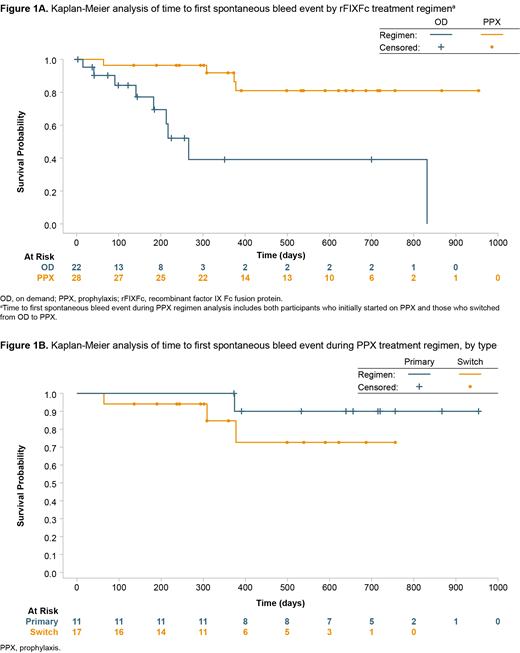
Results: In PUPs B-LONG, 11 participants began on PPX, with 22 participants beginning with OD regimens. Of the 22 who began OD, 17 switched to PPX, for a total of 28 participants on PPX during the study. Six participants discontinued early, for reasons such as withdrawn consent (n=2 [6.1%]), eligibility criteria not fulfilled (n=2 [6.1%]), an adverse event (n=1 [3.0%]), or physician decision (n=1 [3.0%]). A total of 24 spontaneous bleed events occurred. The baseline age of PUPs was not a statistically significant factor for time to first spontaneous bleed event for either regimen or for those who switched from rFIXFc OD to PPX. KM analyses showed that participants receiving PPX had a reduced frequency and extended time to first spontaneous bleed event compared with those receiving OD therapy (Figure 1A). Median (range) time to first spontaneous bleed event was 341.5 (64.0-378.0) days for those on PPX (n=4) and was 183.0 (15.0-832.0) days for those on OD (n=9) who experienced a spontaneous bleed event. Four participants on PPX had first spontaneous bleed events in a joint or in skin/mucosa (n=2 [50%] participants for each location). Nine participants on OD had a first spontaneous bleed event in the skin/mucosa (n=4, 44%), internal or joint bleeds (n=2 [22%] participants for each location), or other locations (n=1 [11%]). Furthermore, during their PPX treatment period, participants on primary PPX had a lower frequency and longer time to first spontaneous bleed event than those who switched from OD to PPX (Figure 1B).
Conclusions: rFIXFc PPX prolonged the time to first spontaneous bleed event and reduced the frequency of first bleed events versus OD in PUPs with hemophilia B. In participants who received primary PPX, the frequency of a first spontaneous bleed event during the study was lower than in those receiving OD treatment initially. Although these results are limited to a small patient group, they suggest that the type of treatment regimen impacts bleed pattern in pediatric PUPs.
Funding: This study was funded by Sanofi and Sobi.
Nolan: Sobi: Other: Personal fees; Bayer: Other: Sponsorship; CSL Behring: Other: Sponsorship; Sanofi: Other: Sponsorship. Recht: Novo Nordisk: Consultancy; Kedrion: Consultancy; Hema Biologics: Consultancy; Genentech: Consultancy; CSL Behring: Consultancy; Catalyst Biosciences: Consultancy; Octapharma: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; uniQure: Consultancy; Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders: Speakers Bureau; American Thrombosis and Hemostasis Network: Current Employment; Oregon Health & Science University: Current Employment. Rendo: Sanofi: Current Employment, Other: May hold shares and/or stock options . Falk: Sobi: Current Employment, Other: May hold shares and/or stock options. Foster: Sanofi: Current Employment, Other: May hold shares and/or stock options in the company. Casiano: Sanofi: Current Employment, Other: May hold shares and/or stock options. Rauch: BioMarin: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Research Funding; LFB: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees. Shapiro: Genentech: Other: Advisory board fees, Research Funding, Speakers Bureau; Glover Blood Therapeutics: Research Funding; Kedrion Biopharma: Research Funding; Novartis: Research Funding; Novo Nordisk: Other: Advisory board fees, Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding; Bioverativ (a Sanofi company): Other: Advisory board fees, Research Funding; BioMarin: Research Funding; Agios: Research Funding; Octapharma: Research Funding; OPKO: Research Funding; Pfizer: Research Funding; Prometric BioTherapeutics: Research Funding; Sangamo: Other: Advisory board fees, Research Funding; Sigilon Therapeutics: Other: Advisory board fees, Research Funding; Takeda: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal